What is Gene Therapy?
Gene therapy is a revolutionary medical approach that aims to treat or prevent disease by modifying a person's genes. Instead of using drugs or surgery, gene therapy works by adding, altering, or removing genetic material to achieve a therapeutic effect. This could involve replacing a faulty gene with a healthy one, inactivating a gene that is causing harm, or introducing a new gene to help fight a disease. The core principle behind gene therapy is to address the root cause of genetic disorders or acquired diseases at the molecular level, offering the potential for long-lasting or even curative treatments for conditions that were previously untreatable. Understanding gene-therapy is essential to appreciating its transformative potential in modern medicine and the ongoing efforts to find gene therapy research.
The First Successful Clinical Use of Gene Therapy: A Turning Point in Medicine
Gene therapy represents a groundbreaking paradigm in modern medicine, offering a fundamentally new approach to treating or preventing diseases by directly addressing their genetic roots. Instead of simply managing symptoms, gene therapy aims to correct, replace, or inactivate faulty genes, or even introduce entirely new genetic material to achieve a therapeutic outcome. This direct manipulation of a patient's genetic blueprint holds immense promise for conditions previously deemed untreatable, providing the potential for long-lasting and in some cases, curative effects. Grasping what is gene-therapy is crucial to understanding its revolutionary impact on healthcare and the tireless efforts made to find gene therapy research.
A Historic Breakthrough: September 1990
The quest for the first successful clinical use of gene therapy was a long and challenging journey, punctuated by scientific breakthroughs and ethical considerations. The pivotal moment arrived on September 14, 1990, when four-year-old Ashanti DeSilva became the inaugural patient to receive gene-therapy. She was suffering from severe combined immunodeficiency (SCID), specifically adenosine deaminase (ADA) deficiency, a devastating genetic disorder that leaves the body virtually defenseless against infections. This landmark treatment, conducted by Drs. W. French Anderson, Michael Blaese, and Kenneth Culver at the National Institutes of Health (NIH) in the US, marked a monumental leap in medical advancements and unequivocally demonstrated the therapeutic potential of genetic manipulation.

The choice of ADA-SCID as the initial target for gene-therapy was strategic. It is a rare, life-threatening condition where a single gene defect leads to a severe immune system collapse. Crucially, the affected T-cells of the immune system are relatively easy to extract from a patient, modify, and then reinfuse, making it an ideal candidate for early-stage gene-therapy trials.
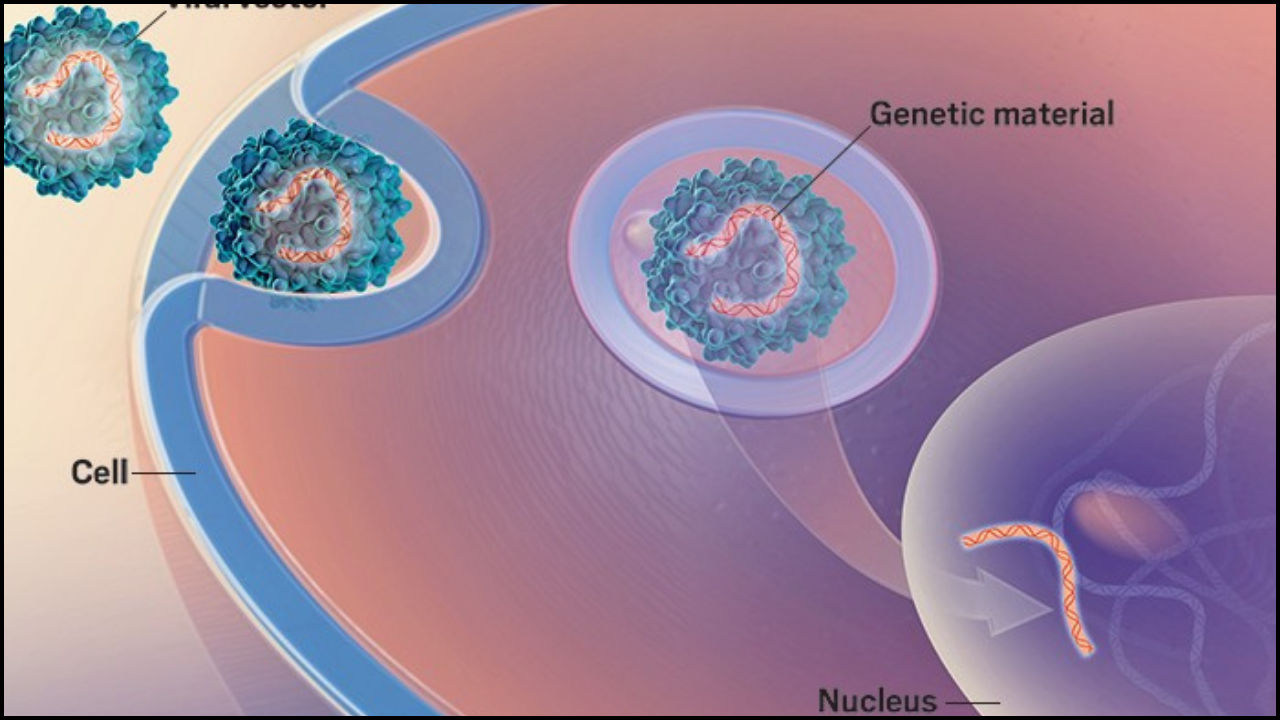
Viral-Vector Gene Therapy: The Retroviral Delivery System
Central to this initial success was the innovative delivery mechanism: viral-vector gene-therapy. This method ingeniously repurposes viruses, which are naturally evolved to efficiently infect cells and deliver their genetic material, as safe and effective vehicles for transporting therapeutic genes. Once stripped of their disease-causing elements, these modified viruses become powerful tools in the gene therapy arsenal.
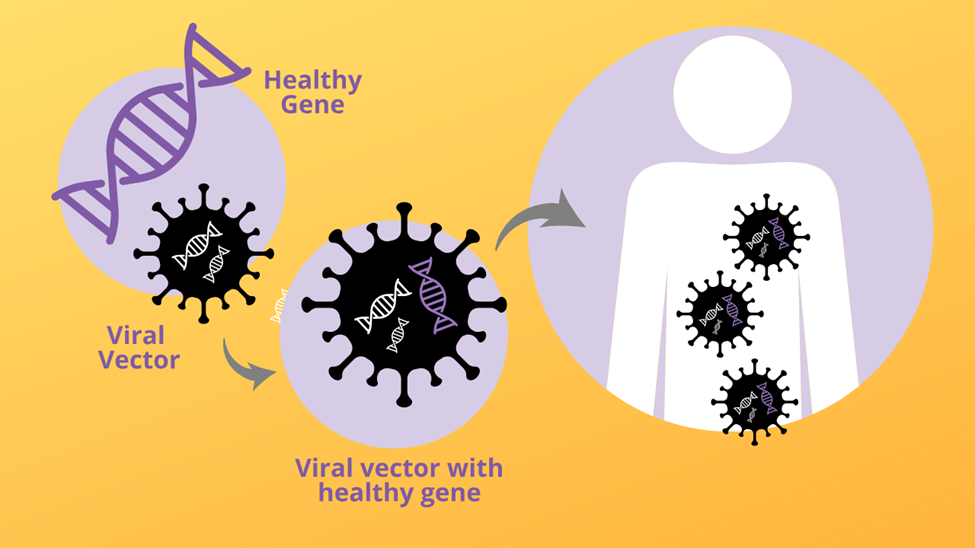
For Ashanti DeSilva's treatment, a disabled retroviral vector was employed. Retroviral vectors, derived from retroviruses (though rendered harmless for therapeutic use), possess a unique and highly advantageous characteristic: their ability to integrate their genetic payload directly into the host cell's genome. This means that the newly introduced, functional ADA gene became a permanent fixture within Ashanti's treated white blood cells. As these cells divided and reproduced, all their daughter cells also carried the corrective gene, ensuring a sustained production of the missing ADA enzyme. The procedure involved extracting some of Ashanti's white blood cells, introducing the functional ADA gene using the retroviral vector, and then returning these genetically modified cells to her body.
Lasting Impact and Expanding Horizons
While Ashanti DeSilva's initial treatment was not an overnight cure and involved continued supportive enzyme replacement therapy, the long-term outcome was undeniably successful. Over time, the genetically modified cells gained traction, leading to a significant and durable improvement in her immune function. She was able to attend school, interact with others, and live a life far removed from the severe limitations imposed by her condition. This profound success served as indisputable proof-of-concept for the viability and potential of gene therapy in humans.
The landmark achievement profoundly impacted the trajectory of medical research and investment in the field. It unequivocally demonstrated that viral-vector gene therapy, particularly with retroviral vectors, could safely and effectively deliver functional genes to patients. Despite initial challenges and setbacks in some subsequent early trials, the foundational success with ADA-SCID provided the impetus for relentless refinement of the technology and a deeper understanding of gene expression and immune responses.
The legacy of the first successful clinical use of gene therapy is vast:
1. Validation of a New Therapeutic Modality
It cemented gene therapy as a legitimate and powerful therapeutic strategy, moving it from hypothesis to clinical reality.
2. Catalyst for Further Research
It ignited a surge in gene therapy clinical trials for a diverse array of genetic disorders, including other forms of SCID, cystic fibrosis, hemophilia, and various neurological conditions.
3. Advancements in Vector Technology
The experience with retroviral vectors spurred intensive medical research into developing safer and more efficient viral-vector gene therapy systems. This led to the creation of lentiviral vectors (a type of retroviral vector capable of infecting non-dividing cells) and adeno-associated virus (AAV) vectors, which are now widely favored for their favorable safety profiles and ability to target specific tissues. These ongoing advancements are vital for any institution seeking to find gene therapy research for new applications.
Retrovir US Medical Research and the Gene Therapy Landscape
The United States has consistently been a global leader in gene therapy research and development, with much of the early pioneering work, including Ashanti DeSilva's treatment, originating from leading US medical research institutions like the NIH.
The phrase “Retrovir US medical research” in the context of gene therapy broadly refers to the extensive historical and ongoing scientific endeavors within the US that have utilized and refined retroviral vectors for therapeutic purposes. It's important to note that while “Retrovir” (zidovudine) is a specific HIV medication, “retroviral” in gene therapy context refers to the class of viruses used as delivery vehicles. US medical research has been instrumental in:
- Refining Vector Safety
- Driving Clinical Trials and Approvals
- Scalable Manufacturing
1. Refining Vector Safety
Developing safer and more targeted retroviral vectors by meticulously removing pathogenic viral genes and ensuring stable integration of therapeutic genes.
2. Driving Clinical Trials and Approvals
Spearheading numerous clinical trials using retroviral and other viral-vector gene therapy approaches, which have directly led to the approval of several gene therapy drugs by the U.S. Food and Drug Administration (FDA). These FDA approvals are a testament to the rigorous medical research and development conducted in the US.
3. Scalable Manufacturing
Pioneering the development of robust and scalable manufacturing processes for viral-vector gene therapy products, a critical step for making these complex treatments accessible to patients.
Conclusion
Today, the US is home to several FDA-approved gene therapy treatments, offering hope for conditions such as spinal muscular atrophy (SMA), specific inherited retinal dystrophies, and certain blood disorders. These approvals are a direct continuation of the pioneering work begun with the first successful clinical use of gene therapy. The relentless pursuit to find gene therapy research and the increasing demand to buy gene therapy papers for a comprehensive understanding highlight the rapid pace and transformative potential of this field within US medical research and globally. The initial success with a retroviral vector marked the dawn of a new era of medical advancements, offering tangible hope for patients grappling with previously untreatable diseases.